Lewis Dot Structure For Vitamin C
Image: Vitamin C
To View the Vitamin C in 3D --->> with Jsmol
Vitamin C
Vitamin C has the chemical formula C6H8O6 and a molecular mass of 176.14 grams per mol. Vitamin C is purely the L-enantiomer of ascorbate; the opposite D-enantiomer has no physiological significance. Both forms are mirror images of the same molecular structure.
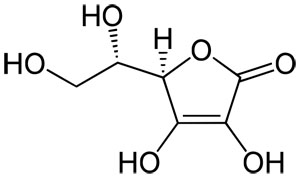 Vitamin C Molecular Structure
Vitamin C Molecular Structure
Discovery and History
The need to include fresh plant food in the diet to prevent disease was known from ancient times. Native peoples living in marginal areas incorporated this into their medicinal lore. For example, infusions of pine needles are used in the arctic zone, or the leaves from species of drought resistant trees in desert areas.
Through history the benefit of plant food for the survival of sieges and long sea voyages was recommended by enlightened authorities. In the seventeenth century Richard Woodall, a ship's surgeon to the British East India Company, recommended the use of lemon juice as a preventive and cure in his book "Surgeon's Mate" The early eighteenth century Dutch writer, Johannes Bachstrom gave the firm opinion that "scurvy is solely owing to a total abstinence from fresh vegetable food, and greens; which is alone the primary cause of the disease."
The first attempt to give scientific basis for the cause of scurvy was by a ship's surgeon in the British Royal Navy, James Lind, who at sea in May 1747 provided some crew members with lemon juice in addition to normal rations while others continued on normal rations alone. In the history of science this is considered to be the first example of a controlled experiment comparing results on two populations of a factor applied to one group only with all other factors the same. The results conclusively showed that lemons prevented the disease. Lind wrote up his work and published it in 1753.
Lind's work was slow to be noticed, partly because he gave conflicting evidence within the book and partly because of social inertia in some elements at the British admiralty who saw care for the well being of ships' crew as a sign of weakness. It was 1795 before the British navy adopted lemon or lime juice as standard issue at sea. (This practice is probably what led to the nickname limey for British people, especially British sailors.)
The name antiscorbutic was used in the eighteenth and nineteenth centuries as general term for those foods know to prevent scurvy even though there was no understanding of the reason for this. As well as lemons, limes and oranges ; sauerkraut, salted cabbage, malt, and portable broth were employed with variable effect. James Cook relied on sauerkraut to prevent the disease on his voyages of exploration. In 1907, Alex Holst and Theodore Frohlich, two Norwegian biochemists studying beriberi contracted aboard ship's crews in the Norwegian Fishing Fleet, wanted a small test mammal to substitute for the pigeons then used. They fed guinea pigs the test diet, which had earlier produced beriberi in their pigeons, and were surprised when scurvy resulted instead. Until that time scurvy had not been observed in any organism apart from humans and was considered a completely human disease.
In 1928 the arctic anthropologist and adventurer Vilhjalmur Stefansson attempted to prove his theory of how Eskimo (Inuit) people are able to avoid scurvy with almost no plant food in their diet. This had long been a puzzle because the disease had struck European arctic explorers living on similar high meat diets. Stefansson theorised that the native peoples of the arctic got their vitamin C from meat and offal that was raw or minimally cooked. Starting in February 1928 for one year he and a colleague lived on an animal flesh only diet under medical supervision at New York's Bellevue Hospital and remained healthy.
In the early twentieth century, the Polish American scientist Casimir Funk conducted research into deficiency diseases and in 1912 formulated the concept of vitamins, for the elements in food which are essential to health. Then, from 1928 to 1933, the Hungarian research team of Joseph L Svirbely and Albert Szent-Gyorgyi, and independently the American Charles Glen King, first isolated vitamin C and showed it to be ascorbic acid.
In 1933/1934, the British chemists Sir Walter Norman Haworth and Sir Edmund Hirst, and independently the Polish Tadeus Reichstein, succeeded in synthesizing the vitamin, the first to be artificially produced. This made possible the cheap mass production of vitamin C. Haworth was awarded the 1937 Nobel Prize for Chemistry largely for this work.
In 1959 the American J.J. Burns showed that the reason why some mammals were susceptible to scurvy was due to the inability of their livers to produce the active enzyme L-gulonolactone oxidase, which is the last of the chain of four enzymes which synthesise ascorbic acid.
Citrus fruits (lime, lemon, orange, grapefruit) and tomatoes are good common sources of vitamin C. Other foods that are good sources of vitamin C include papaya, broccoli, brussels sprouts, blackcurrants, strawberries, cauliflower, spinach, cantaloupe, and kiwifruit.
The amount of vitamin C in foods of plant origin depends on:
Most species of animals synthesise their own vitamin C. It is therefore not a vitamin for them. Synthesis is achieved through a sequence of enzyme driven steps, which convert glucose to ascorbic acid. It is carried out either in the kidneys, in reptiles and birds, or the liver, in mammals and perching birds. The loss of an enzyme concerned with ascorbic acid synthesis has occurred quite frequently in evolution and has affected most fish, many birds; some bats, guinea pigs and most but not all primates, including Man. The mutations have not been lethal because ascorbic acid is so prevalent in the surrounding food sources. For example an adult goat can internally manufacture more than 13,000 mg of vitamin C per day in normal health and as much as 100,000 mg daily when faced with life-threatening disease. It was only realised in the 1920s that some cuts of meat and fish are also a source of vitamin C for humans. The muscle and fat which make up the modern western diet are however poor sources. As with fruit and vegetables cooking destroys the vitamin C content. Vitamin C is produced from glucose by two main routes. The Reichstein process developed in the 1930s uses a single pre-fermentation followed by a purely chemical route. The more modern Two-Step fermentation process was originally developed in China in the 1960s, uses additional fermentation to replace part of the later chemical stages. Both processes yield approximately 60% vitamin C from the glucose feed. In 1934, the Swiss pharmaceutical company Hoffmann-La Roche was the first to mass produce synthetic vitamin C, under the brand name of Redoxon. Main producers today are BASF/ Takeda, Roche, Merck and the China Pharmaceutical Group Ltd of the People's Republic of China. Functions of vitamin C in the body As a participant in hydroxylation, vitamin C is needed for the production of collagen in the connective tissue. These fibres are ubiquitous throughout the body; providing firm but flexible structure. Some tissues have a greater percentage of collagen, especially: skin , mucous membranes , teeth and bones. Vitamin C is required for synthesis of dopamine, noradrenaline and adrenaline in the nervous system or in the adrenal glands. Vitamin C is also needed to synthesise carnitine, important in the tranfer of energy to the cell mitochondria. It is a strong antioxidant. The tissues with greatest percentage of vitamin C — over 100 times the level in blood plasma — are the adrenal glands, pituitary , thymus , corpus luteum, and retina. The brain, spleen, lung, testicle, lymph nodes, liver, thyroid, small intestinal mucosa, leukocytes, pancreas, kidney and salivary glands usually have 10 to 50 times the concentration present in plasma. >Lack of ascorbic acid in the daily diet leads to a disease called scurvy, a form of avitaminosis that is characterized by: A healthy person on a balanced western diet should be able to get all the vitamin C needed to prevent the symptoms of scurvy from their daily diet. People who smoke, those under stress and women in pregnancy have a slightly higher requirement. The amount of vitamin C needed to avoid deficiency symptoms and maintain health has been set by variously national agencies as follows:- 4O mg per day UK Food Standards Agency 60-95 mg per day US Food and Nutrition Board 2001 revision. Some researchers have calculated the amount needed for an adult human to achieve similar blood serum levels as Vitamin C synthesising mammals as follows:- 200 mg per day - Linus Pauling Institute & US National Institutes of Health (NIH) Recommendation. 3000 mg per day - Vitamin C Foundation's recommendation. 6000-12000 mg per day – Thomas Levy , Colorado Integrative Medical Centre recommendation. 6000-18000 mg per day - Linus Pauling's daily recommendation The small size of the ascorbic acid molecule means the kidneys cannot retain it in the body. Quite a low level in the blood serum will cause traces to be present in the urine. All vitamin C synthesising mammals have traces in the urine at all times. In April 1998 Nature reported alleged carcinogenic and teratogenic effects of excessive doses of vitamin C. This was given great prominence in the world's media. The effects were noted in test tube experiments and on only two of the 20 markers of free radical damage to DNA. They have not been supported by further evidence from living organisms. Almost all mammals manufacture their own vitamin C in amounts equivalent to human doses of thousands of milligrams per day. Large amounts of the vitamin are used in orthomolecular medicine and no harmful effects have been observed even in doses of 10,000 mg per day or more.
Artificial chemical synthesis
Vitamin C deficiency
Daily requirement
Lewis Dot Structure For Vitamin C
Source: https://www.worldofmolecules.com/antioxidants/vitaminc.htm

0 komentar:
Posting Komentar